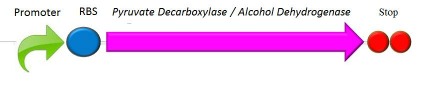Team:BioscienceDragons AZ/Project/Ethanol Generator
From 2013hs.igem.org
| Line 31: | Line 31: | ||
Our project is building off the ethanol generator constructed by the Nevada iGEM Team in 2011. The part was generously shared with us and it will be the second plasmid we transform into the E. coli after having transformed it with proteorhodopsin. This second gene will allow the cell to ferment glucose into ethanol which combined with proteorhodopsin will allow for the cell to live of light rather than media and solely use glucose to ferment it into ethanol. The generator includes the coding sequence for two separate enzymes, PDC (Pyruvate Decarboxylase) and ADH (Alcohol Dehydrogenase). | Our project is building off the ethanol generator constructed by the Nevada iGEM Team in 2011. The part was generously shared with us and it will be the second plasmid we transform into the E. coli after having transformed it with proteorhodopsin. This second gene will allow the cell to ferment glucose into ethanol which combined with proteorhodopsin will allow for the cell to live of light rather than media and solely use glucose to ferment it into ethanol. The generator includes the coding sequence for two separate enzymes, PDC (Pyruvate Decarboxylase) and ADH (Alcohol Dehydrogenase). | ||
| - | The process begins with the intake of glucose by the cell. Glucose is broken down into pyruvic acid through the process of hydrolysis. The enzyme, PDC, then goes on to catalyze the decarboxylation of pyruvic acid. Decarboxylation is basically the removal of a carboxyl group and which results in the release of carbon dioxide. So in the case of pyruvic acid, decarboxylation will produce acetaldehyde and carbon dioxide. The second enzyme, ADH, then goes on to reduce acetaldehyde into ethanol using the electron donor, NADH. The final result is the production of ethanol and carbon dioxide from glucose and the oxidation of NADH to NAD+. The theoretical yield should be about 25 g/mL of ethanol per 50 g/mL of glucose, a ratio of about 1:2. With these two parts transformed the next steps can be taken in the development of a biopanel (see more on [[Team:BioscienceDragons_AZ/Human Practices/Bioscience High School|Human Practices). | + | The process begins with the intake of glucose by the cell. Glucose is broken down into pyruvic acid through the process of hydrolysis. The enzyme, PDC, then goes on to catalyze the decarboxylation of pyruvic acid. Decarboxylation is basically the removal of a carboxyl group and which results in the release of carbon dioxide. So in the case of pyruvic acid, decarboxylation will produce acetaldehyde and carbon dioxide. The second enzyme, ADH, then goes on to reduce acetaldehyde into ethanol using the electron donor, NADH. The final result is the production of ethanol and carbon dioxide from glucose and the oxidation of NADH to NAD+. The theoretical yield should be about 25 g/mL of ethanol per 50 g/mL of glucose, a ratio of about 1:2. With these two parts transformed the next steps can be taken in the development of a biopanel (see more on [[Team:BioscienceDragons_AZ/Human Practices/Bioscience High School|Human Practices]]). |
[[File:EG.jpg|center]] | [[File:EG.jpg|center]] | ||
Revision as of 21:31, 21 June 2013
Ethanol Generator
Our project is building off the ethanol generator constructed by the Nevada iGEM Team in 2011. The part was generously shared with us and it will be the second plasmid we transform into the E. coli after having transformed it with proteorhodopsin. This second gene will allow the cell to ferment glucose into ethanol which combined with proteorhodopsin will allow for the cell to live of light rather than media and solely use glucose to ferment it into ethanol. The generator includes the coding sequence for two separate enzymes, PDC (Pyruvate Decarboxylase) and ADH (Alcohol Dehydrogenase).
The process begins with the intake of glucose by the cell. Glucose is broken down into pyruvic acid through the process of hydrolysis. The enzyme, PDC, then goes on to catalyze the decarboxylation of pyruvic acid. Decarboxylation is basically the removal of a carboxyl group and which results in the release of carbon dioxide. So in the case of pyruvic acid, decarboxylation will produce acetaldehyde and carbon dioxide. The second enzyme, ADH, then goes on to reduce acetaldehyde into ethanol using the electron donor, NADH. The final result is the production of ethanol and carbon dioxide from glucose and the oxidation of NADH to NAD+. The theoretical yield should be about 25 g/mL of ethanol per 50 g/mL of glucose, a ratio of about 1:2. With these two parts transformed the next steps can be taken in the development of a biopanel (see more on Human Practices).
 "
"