Team:NC School of Sci Math/Lab Notebook
From 2013hs.igem.org
Jack Allen (Talk | contribs) |
Jack Allen (Talk | contribs) |
||
| Line 124: | Line 124: | ||
<h4><a style="color:#00008B;" name="03/18/2013">03/18/2013</a></h4> | <h4><a style="color:#00008B;" name="03/18/2013">03/18/2013</a></h4> | ||
</html> | </html> | ||
| - | <p><b> The following models have been created</b> | + | <p><b> The following models have been created</b></p> |
| + | * We should use these in the proposal, but continue to work on the Tinkercell model | ||
[[File:Design.jpg|700px]] | [[File:Design.jpg|700px]] | ||
| - | + | <html> | |
| - | < | + | <h4><a style="color:#00008B;" name="03/25/2013">03/25/2013</a></h4> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | |
</html> | </html> | ||
| - | + | <p><b>Everyone began editing the proposal on a Google Doc today</b></p> | |
| - | + | *Want to be finished by March 29th | |
| - | + | *Tinkercells will probably not be ready in time | |
| - | + | **Having a lot of trouble getting fluorescent protein levels to zero when they should be | |
| - | + | **Danny will talk to Morgan to see if he can help | |
| - | + | *Edit Edit Edit! | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | * | + | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<html> | <html> | ||
<br/> | <br/> | ||
Revision as of 15:37, 18 June 2013
Home Team Project Details Lab Notebook Results Human Impact Biosafety Acknowledgments Official Team Profile
Planning and Development
01/11/2013
Brainstorming for project ideas- LDL-cholesterol biosensor
- Ocean salinity regulator (especially for coral reefs)
- Water contaminant multibiosensor
More research should be done
- Find related projects that have been completed
- Obstacles?
- BioBricks?
- Presentations in 3-4 weeks
01/18/2013
Discussions of each project- LDL-cholesterol biosensor
- Could be used as a blood test
- Blood sugar monitor-like device
- Hard to measure any color change in blood
- Ocean salinity regulator (especially for coral reefs)
- Need a lot of bacteria
- Environmental concerns
- Bacteria can do well in coral reefs
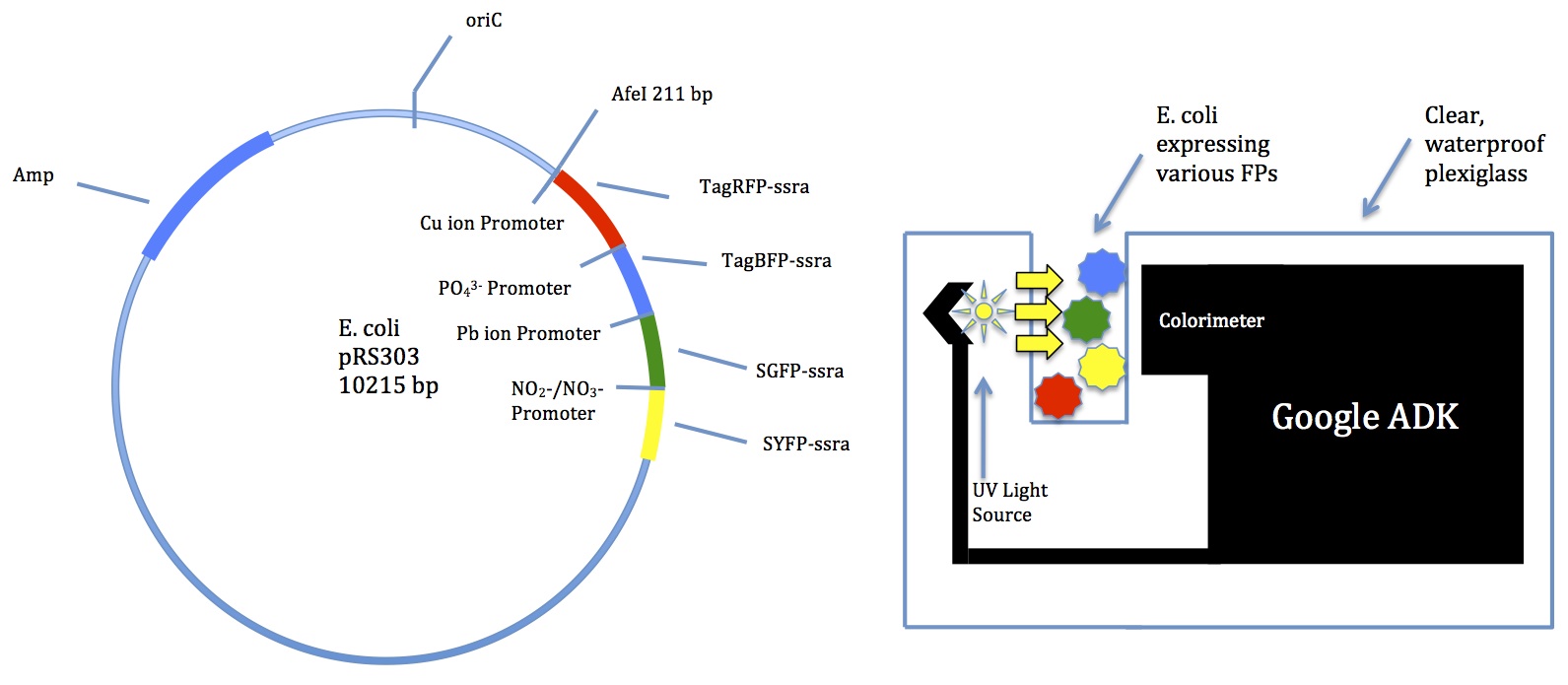
- Water contaminant multibiosensor
- Several Biobrick promoters for common contaminants
- Usage in sewer tanks
- Many different colors of fluorescent proteins
- Could incorporate newer technology for better color-sensing
- Will look up data on promoter/FP expression
02/01/2013
Presentations
- All ideas sound promising
- Meet next week to decide which idea to pursue
02/08/2013
Decision on project- LDL-cholesterol idea won't work
- Costly and hard to detect FPs in blood
- Multibiosensor is appealing
- Many applications
- Expandable with many different FPs
- Could bring in new technology
- Ocean salinity regulator is difficult because of the ocean
- Environmental factors and unknown variables
Multibiosensor chosen
02/15/2013
A few members met with Mr. Jon Davis to discuss potential technology
- Suggested a Google device called ADK
- Comes with a colorimeter
- Can be programmed for many functions
- School has a few we could try out
02/22/2013
Goals for the immediate future
- Find Biobricks
- Set up schedule for lab work
- Further research into possible uses
- Preliminary lab experiments
Project Proposal
03/01/2013
Discussion of upcoming project proposal:
- Introduction, Solution Statement, and Methods
- Need to find out some background information
- Should make use of Tinkercell modeling
03/04/2013
Assignments of roles for the upcoming project proposal:
- Madeline will investigate background information
- Danny will work on Tinkercell models
- Jack will write the solution statement and methods sections
- Synthesize our writing on a Google Doc
- Try to have individual parts done in about 2 weeks
03/18/2013
The following models have been created
- We should use these in the proposal, but continue to work on the Tinkercell model
03/25/2013
Everyone began editing the proposal on a Google Doc today
- Want to be finished by March 29th
- Tinkercells will probably not be ready in time
- Having a lot of trouble getting fluorescent protein levels to zero when they should be
- Danny will talk to Morgan to see if he can help
- Edit Edit Edit!

Fig. 1 Colony-PCR screen of precA-GFP construct. No detectable shift of any screened clone can be seen compared to the control. Screen has to be repeated.

Fig. 2 Colony-PCR screen of precA-LacZ construct. Most clones show a small shift upwards in product length. Furthermore, they show a second band at 1200 bp, probably due to unspecific primer binding.

Fig. 3 Colony-PCR screen of psulA-GFP (#3.1-3.8) and psulA-LacZ (#4.1-4.8). Only clones containing the psulA promoter can give a PCR product of 1000 bp (psulA_GFP) or 3700 bp (psulA_LacZ). Positive clones are i.e. #3.4, #4.2, #4.8.
The screening gave positive clones for all constructs (fig. 2, fig. 3), but not the precA_GFP construct (fig. 1). Therefore this screen has to be repeated using a larger number of colonies.
- 5 ml LB-Amp overnight cultures were inoculated for clones #2.3, #3.4 and #4.8.
03/15/2012
- The colony-PCR for the precA_GFP construct was repeated, now screening a number of 31 colonies. As control, again the amplicon of the original GFP biobrick was used.

Fig. 1 Repetition of colony-PCR for precA_GFP construct. Clones #6, 18 and 26 were positive (shift in amplicon length of ~200 bp).
3 positive colonies were detected, as they show a shift in band size of ~ 200 bp as expected by the introduction of the recA-promoter. Those were clones # 6, 18 and 26.
- O/n LB-amp cultures were inoculated for clones #18 and #26.
- Miniprep of the o/n cultures inoculated the day before for clones #2.3, 3.4 and 4.8 were done.
- concentrations measured using Nanodrop are all in between 70 and 140 ng/µl
- concentrations measured using Nanodrop are all in between 70 and 140 ng/µl
- Test digestions were performed in order to detect, whether the constructs would give the expected band patterns on the gel.
- #2.3 with EcoRI/BglI; as control the original LacZ biobrick was digested EcoRI/BgII as well
- #3.4 and #4.8 were digested NotI in order to see, whether an insert is present

Fig. 2 Test digestions for the constructs recA_LacZ (#2.3), psulA_GFP (#3.4) and sulA_LacZ (#4.8). All constructs digested showed the right bands (left gel compared to virtual gel with expected bands on the right). Construct #2.3 gave the expected shift of the lowest band (arrow) compared to the LacZ-control construct (original biobrick BBa_K173004) due to the presence of the precA promoter.
03/16/2012
- Miniprep of precA_GFP clones #18 and #26 inoculated the previous
- Measurement of DNA concentration with the Nanodrop gave 71 ng/ul for both constructs
- Measurement of DNA concentration with the Nanodrop gave 71 ng/ul for both constructs
- Test digestion of 1 ug of #18 and #26 with EcoRI/NcoI
- Band correct for both constructs

- All constructs (clones #1.18, 2.3, 3.4, 4.8) were send for sequencing to GATC using standard biobrick sequencing primer VF2
03/17/2012
- Sequencing results were obtained from GATC for the first 4 Biobricks we did
- Sequence was confirmed for all 4 constructs; cloning was successful!
| Part | DNA Confirmation |
| RecA-GFP | OK |
| RecA-LacZ | OK |
| SulA-GFP | OK |
| SulA-LacZ | OK |
05/08/2012
Anealing of oligos for precB and precC:
Got oligo sequences from pubmed (looked for appropriate ATG and then took the following 70 basepairs as promoter sequence)
RecB:
fwd.:
aattcgcggccgcttctagagCCTGAAGGCTGGAAAGTGTGGGAGAACGTCAGCGCGTTGCAGCAAACAATGCCCCTGATGAGTGAAAAGAc
rev.:
ctaggTCTTTTCACTCATCAGGGGCATTGTTTGCTGCAACGCGCTGACGTTCTCCCACACTTTCCAGCCTTCAGGctctagaagcggccgcg
RecC:
fwd.:
aattcgcggccgcttctagagTTCACCCGGGGGCAGAGAAGGCGAGATGACCCGCCTGCATTGCCCGAATCGTCAGTAGTCAGGAGCCGCTc
rev.:
ctaggAGCGGCTCCTGACTACTGACGATTCGGGCAATGCAGGCGGGTCATCTCGCCTTCTCTGCCCCCGGGTGAActctagaagcggccgcg
Anealing-Protocol:
- 5 ul of each oligo (diluted to a concentration of 100 mM)
- 5 ul of NEB buffer 2
- 35 ul of water
- Mix
- Heat up reaction mix to 95 °C for 5 minutes
- Cool down slowly to room temperature (then store at -20 °C)
Ligation precB_LacZ / precC_lacZ:
Add contents in the following order:
- 12 ul water
- 2 ul T4 DNA ligase buffer
- 4 ul LacZ-backbone
- 1 ul precB / precC
- 1 ul T4 ligase
- leave 20 minutes at room temperature
- heat up to ca. 70°C to destroy ligase-enzymes
Transformation of precB_LacZ / precC_LacZ into Top10:
- Transformation-protocoll missing!
05/26/2012
Colony PCR:
Screening of the two different biobrick clonings done by colony-PCR.
Protocol:
A reaction mix was prepared containing 0.2 µl of each screening primer used for the screening, 9.6 µl of water and 10 µl of 2x PCR mastermix (fermentas). Finally, a colony was picked from a plate using a sterile pipette tip and was dipped into the PCR mix a few times. 12 clones were screened for each different cloning setup as follows:
precB_LacZ: primers VF2: and precB Reverse: were used. Only in case precB was successfully cloned into the backbone containing the reporter gene, we would get a PCR product.
precC_LacZ: primers VF2 and VR (standard sequencing primers) were used. We compared the product size of the different clones to the product size from the original vectors from the registry (only containing precA or LacZ). In case the cloning was successful, we should see a 200 bp shift in product size, which can be detected by gel electrophoresis.
The PCR program was done as follows:
94°C/3min
 "
"