Team:NC School of Sci Math/Results
From 2013hs.igem.org
Jack Allen (Talk | contribs) |
Jack Allen (Talk | contribs) |
||
| (6 intermediate revisions not shown) | |||
| Line 10: | Line 10: | ||
Content= | Content= | ||
__NOTOC__ | __NOTOC__ | ||
| - | ==Results and Discussion== | + | ===Results and Discussion=== |
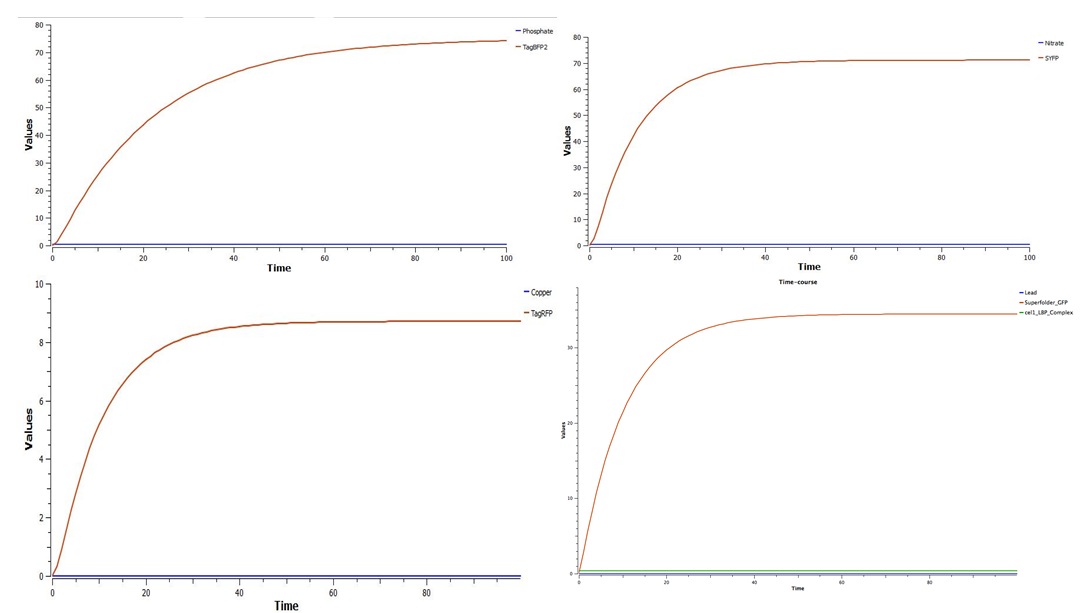
| - | In conclusion, our results demonstrate that the biopart created is capable of sensing the targeted analytes. As shown in the graphs below, fluorescent protein concentration increases rapidly in the first few minutes after exposure to the analyte. As protein degradation and translation reach equilibrium, the level of protein remains constant. As Tinkercell incorporates cellular processes such as mRNA degradation, movement within a cell, and transcription rates, the model is highly accurate. For the initial concentrations of each substance, the lowest level considered “dangerous for human consumption” by the EPA was used. For lead, this level was 15 parts per billion, and thus this concentration was used. This was the same for phosphates (100 parts per million), nitrates (10 parts per million), and copper (1.3 parts per million). <br> | + | In conclusion, our results demonstrate that the biopart created is capable of sensing the targeted analytes. As shown in the graphs below, fluorescent protein concentration increases rapidly in the first few minutes after exposure to the analyte. As protein degradation and translation reach equilibrium, the level of protein remains constant. As Tinkercell incorporates cellular processes such as mRNA degradation, movement within a cell, and transcription rates, the model is highly accurate. For the initial concentrations of each substance, the lowest level considered “dangerous for human consumption” by the EPA was used. For lead, this level was 15 parts per billion, and thus this concentration was used. This was the same for phosphates (100 parts per million), nitrates (10 parts per million), and copper (1.3 parts per million). Fluorescent protein concentrations are given in micromolar. <br> |
| - | <div style="margin | + | <div style="margin:15px 0px 10px 0px;">[[File:4graphs.jpg|center|900px]]</div> |
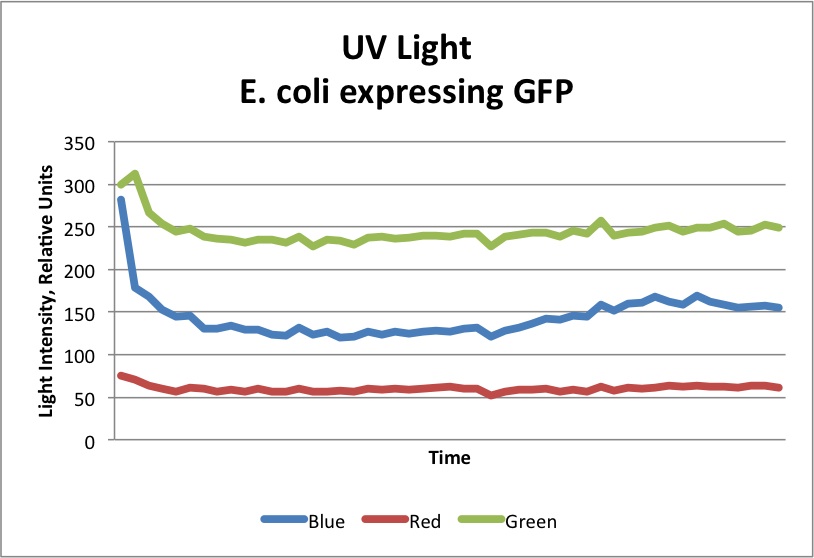
| - | As shown above, even a single cell is capable of increasing fluorescent protein concentration to several micromolar. In tests with the ADK, a concentration of 0.1 Molar was detectable by the ADK. With many cells in local environment reacting to the presence of one of the targeted substances, it can be concluded that enough fluorescence would be produced to activate the ADK response. The graphs below demonstrate the results of lab tests using GFP (about 2/3 as bright as the Superfolder GFP used in the actual biopart) at a concentration of 0.1 Molar. As shown, the detection of green light increased significantly, to the point where the ADK would register a change in light | + | As shown above, even a single cell is capable of increasing fluorescent protein concentration to several micromolar. In tests with the ADK, a concentration of 0.1 Molar was detectable by the ADK. With many cells in local environment reacting to the presence of one of the targeted substances, it can be concluded that enough fluorescence would be produced to activate the ADK response. The graphs below demonstrate the results of lab tests using GFP (about 2/3 as bright as the Superfolder GFP used in the actual biopart) at a concentration of 0.1 Molar. As shown, the detection of green light increased significantly, to the point where the ADK would register a change in light. <br> |
| - | [[File:pGLO.jpg|500 px|center]] < | + | <div style="margin:15px 0px;">[[File:pGLO.jpg|500 px|center]]</div> |
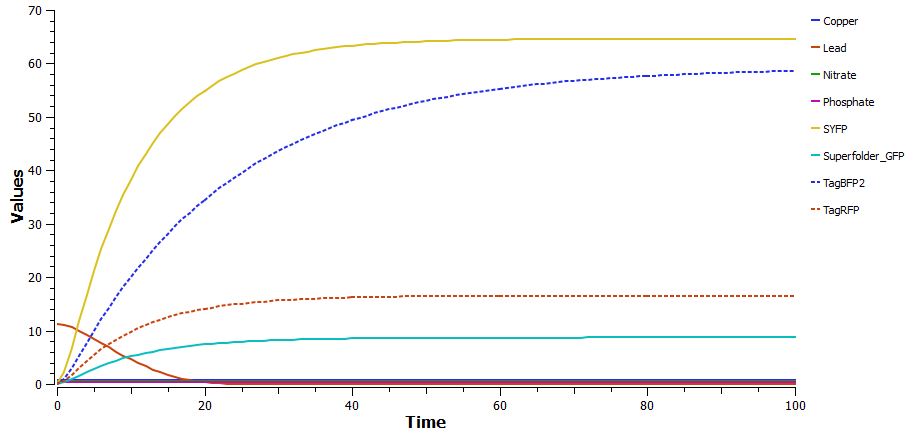
Although separating each part of the biosensor is important for testing, it is perhaps more important that the part be able to function in an environment that contains various levels of many analytes. As shown below, the biosensor was able to effectively regulate fluorescent protein production even in the presence of all four analytes at different concentrations. An unintended result of the system, our biopart actually decreases lead concentration as it binds to the Lead Binding Protein and activates Superfolder GFP production. <br> | Although separating each part of the biosensor is important for testing, it is perhaps more important that the part be able to function in an environment that contains various levels of many analytes. As shown below, the biosensor was able to effectively regulate fluorescent protein production even in the presence of all four analytes at different concentrations. An unintended result of the system, our biopart actually decreases lead concentration as it binds to the Lead Binding Protein and activates Superfolder GFP production. <br> | ||
| - | [[File:Results.jpg|600 px|center]] | + | <div style="margin:15px 0px;">[[File:Results.jpg|600 px|center]]</div> |
| - | In conclusion, our biopart was able to effectively and efficiently detect concentrations of potentially harmful substances at concentrations as low as 15 parts per billion, and produce enough protein for detection by the ADK colorimeter. Therefore, the system has clear applications for detection of all four substances tested in sewer systems, septic tanks, water treatment facilities and in industrial settings. | + | In conclusion, our biopart was able to effectively and efficiently detect concentrations of potentially harmful substances at concentrations as low as 15 parts per billion, and produce enough protein for detection by the ADK colorimeter. Therefore, the system has clear applications for detection of all four substances tested in sewer systems, septic tanks, water treatment facilities and in industrial settings.<br> |
In the future, the biopart could be expanded to detect more substances, even to the point of becoming a nearly-universal reporter for potentially harmful substances. This would obviously extend the applications even further, using synthetic biology for detection of nearly any substance. | In the future, the biopart could be expanded to detect more substances, even to the point of becoming a nearly-universal reporter for potentially harmful substances. This would obviously extend the applications even further, using synthetic biology for detection of nearly any substance. | ||
}} | }} | ||
Latest revision as of 04:11, 22 June 2013
Home Team Project Details Lab Notebook Results Human Impact Biosafety Acknowledgments Official Team Profile
Results and Discussion
In conclusion, our results demonstrate that the biopart created is capable of sensing the targeted analytes. As shown in the graphs below, fluorescent protein concentration increases rapidly in the first few minutes after exposure to the analyte. As protein degradation and translation reach equilibrium, the level of protein remains constant. As Tinkercell incorporates cellular processes such as mRNA degradation, movement within a cell, and transcription rates, the model is highly accurate. For the initial concentrations of each substance, the lowest level considered “dangerous for human consumption” by the EPA was used. For lead, this level was 15 parts per billion, and thus this concentration was used. This was the same for phosphates (100 parts per million), nitrates (10 parts per million), and copper (1.3 parts per million). Fluorescent protein concentrations are given in micromolar.
As shown above, even a single cell is capable of increasing fluorescent protein concentration to several micromolar. In tests with the ADK, a concentration of 0.1 Molar was detectable by the ADK. With many cells in local environment reacting to the presence of one of the targeted substances, it can be concluded that enough fluorescence would be produced to activate the ADK response. The graphs below demonstrate the results of lab tests using GFP (about 2/3 as bright as the Superfolder GFP used in the actual biopart) at a concentration of 0.1 Molar. As shown, the detection of green light increased significantly, to the point where the ADK would register a change in light.
Although separating each part of the biosensor is important for testing, it is perhaps more important that the part be able to function in an environment that contains various levels of many analytes. As shown below, the biosensor was able to effectively regulate fluorescent protein production even in the presence of all four analytes at different concentrations. An unintended result of the system, our biopart actually decreases lead concentration as it binds to the Lead Binding Protein and activates Superfolder GFP production.
In conclusion, our biopart was able to effectively and efficiently detect concentrations of potentially harmful substances at concentrations as low as 15 parts per billion, and produce enough protein for detection by the ADK colorimeter. Therefore, the system has clear applications for detection of all four substances tested in sewer systems, septic tanks, water treatment facilities and in industrial settings.
In the future, the biopart could be expanded to detect more substances, even to the point of becoming a nearly-universal reporter for potentially harmful substances. This would obviously extend the applications even further, using synthetic biology for detection of nearly any substance.
 "
"